What It Is and How It Works About Gas Chromatography
Uses of Gas Chromatography
GC is used as one test to help identify components of a liquid mixture and determine their relative concentration. It may also be used to separate and purify components of a mixture. Chromatography Additionally, gas chromatography can be used to determine vapor pressure, heat of solution, and activity coefficients. Industries often use it to monitor processes to test for contamination or ensure a process is going as planned. Chromatography can test blood alcohol, drug purity, food purity, and essential oil quality. GC may be used on either organic or inorganic analytes, but the sample must be volatile. Ideally, the components of a sample should have different boiling points.
How Gas Chromatography Works
First, a liquid sample is prepared.
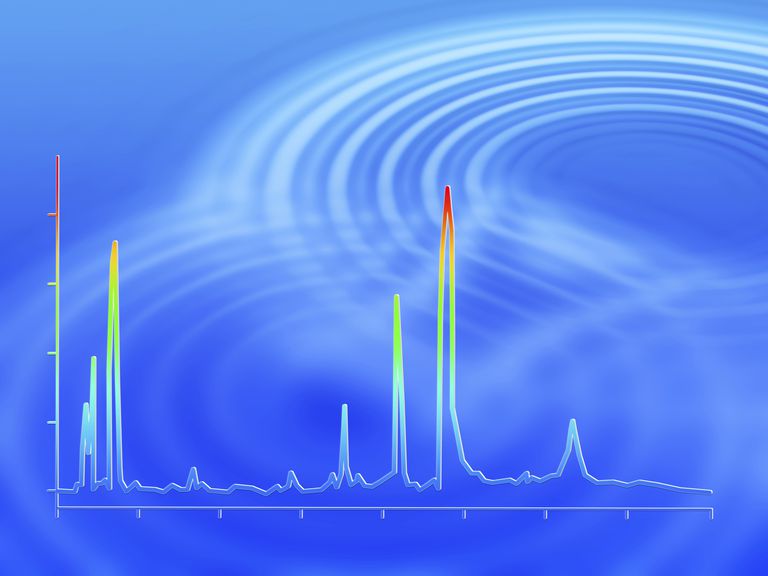
The sample is mixed with a solvent and is injected into the gas chromatograph. Typically the sample size is small -- in the microliters range. Although the sample starts out as a liquid, it is vaporized into the gas phase. An inert carrier gas is also flowing through the chromatograph. This gas shouldn't react with any components of the mixture.Common carrier gases include argon, helium, and sometimes hydrogen. The sample and carrier gas are heated and enter a long tube, which is typically coiled to keep the size of the chromatograph manageable. The tube may be open (called tubular or capillary) or filled with a divided inert support material (a packed column). The tube is long to allow for a better separation of components. At the end of the tube is the detector, which records the amount of sample hitting it. In some cases, the sample may be recovered at the end of the column, too. Autosampler the signals from the detector are used to produce a graph, the chromatogram, which shows the amount of sample reaching the detector on the y-axis and generally how quickly it reached the detector on the x-axis (depending on what exactly the detector detects). The chromatogram shows a series of peaks. The size of the peaks is directly proportional to the amount of each component, although it can't be used to quantify the number of molecules in a sample. Usually, the first peak is from the inert carrier gas and the next peak is the solvent used to make the sample. Subsequent peaks represent compounds in a mixture. In order to identify the peaks on a gas chromatogram, the graph needs to be compared a chromatogram from a standard (known) mixture, to see where the peaks occur.
At this point, you may be wondering why the components of the mixture separate while they are pushed along the tube. The inside of the tube is coated with a thin layer of liquid (the stationary phase). Gas or vapor in the interior of the tube (the vapor phase) moves along more quickly than molecules that interact with the liquid phase. Compounds that interact better with the gas phase tend to have lower boiling points (are volatile) and low molecular weights, while compounds that prefer the stationary phase tend to have higher boiling points or are heavier. Other factors that affect the rate at which a compound progresses down the column (called the elution time) include polarity and the temperature of the column. Because temperature is so important, Analysis it is usually controlled within tenths of a degree and is selected based on the boiling point of the mixture.